Yves Pommier, Mirit I. Aladjem and Kurt W. Kohn
Implemented by : Margot Sunshine
To read the original paper describing this map and the symbols used, click here. This map has been updated since the article was published.
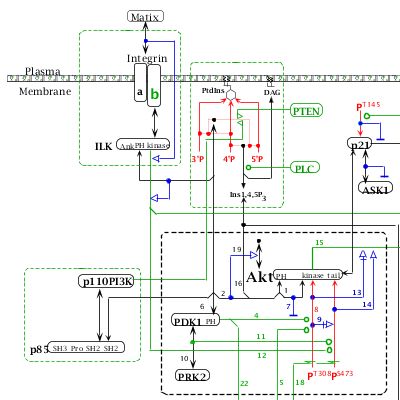
AKT is the cellular homolog of the retroviral oncogene v-Akt. The AKT family comprises three members (AKT1, AKT2 and AKT3), which, in general, are broadly expressed (Vivanco and Sawyers, 2002). AKT is activated by phosphatidylinositol 3-phosphate (PIP3), which is produced by PI(3)kinases (PI3K). As schematized in the MIM, PI3K heterodimers contain both a catalytic (p110) and a regulatory (p85) subunit. PI3K catalyses the phosphorylation of inositol lipids at the D3 position of the inositol ring (#4; B4). The resulting PIP3 activates a variety of downstream effectors, including AKT (#9; D4-5). For example, in response to survival factors such as hematopoietic growth factors (e.g., IL-3), AKT is recruited to the plasma membrane by its binding to PIP3, and is activated by sequential phosphorylation on Thr308 by PDK1 (phosphoinositide-dependent protein kinase-1) (#7; E4-5) and on Ser473 by PDK2 (#10; E4-5) or ILK (#15; E4-5) (Datta et al., 1999; Hill and Hemmings, 2002; Vivanco and Sawyers, 2002; Troussard et al., 2003).
AKT is negatively regulated by the lipid phosphatases PTEN (#20; B3), SHIP-1 and SHIP-2 (#21; B5), which prevent AKT activation by decreasing PIP3 levels (Damen et al., 1996; Stambolic et al., 1998; Wisniewski et al., 1999). Also, the protein phosphatase 2A (PP2A) inactivates AKT directly by dephosphorylating Ser473 and Thr308 (#19; E4 & F4); Ser473 being a better substrate than Thr308 (Andjelkovic et al., 1999). Carboxyl-terminal modulator protein (CTMP) binds to the carboxyl-terminal regulatory domain of AKT at the plasma membrane and reduces the activity of AKT by inhibiting phosphorylation on Ser473 and to a lesser extent Thr308 (#22; E5 & F5) (Maira et al., 2001). Conversely, HSP90 maintains the AKT activity by binding to AKT (#23; D3) and by preventing PP2A-dependent dephosphorylation of AKT (Sato et al., 2000). HSP90 also prevents proteasome-dependent degradation of PDK1 (#24; D2) (Fujita et al., 2002). AKT is therefore regulated by a complex network of phosphorylation/dephosphorylation reactions, which can be concisely summarized in the molecular interaction map.